Considering probiotics for health and performance?
Introduction
The prevalence of digestive disorders has risen significantly over the past 15 years. Digestive disorders are estimated to affect nearly 35% of Americans with a higher prevalence in women and seniors (Everhart et al., 2009). The most common symptoms associated with poor digestion include heart burn, diarrhea, gas, cramping, bloating, constipation, and bad breath. With an increase in the public’s awareness of digestive maladies and the importance of digestive health and increase products aimed at improving various elements of digestive health has also increased. A quick google.com search of “colon health” retrieves a variety of supplements, diets, fasts, and internal therapies touting beneficial results. One of the most popular, however least understood, method of improving digestive health is by supplementing with probiotics. In order to better educate the consumer regarding the potential health benefits to improving the digestive function, a brief understanding of the intestinal ecosystem is needed.
Gut Flora
The intestinal system (gut) provides us with a completely isolated and expansive surface area where outside material is processed and either absorbed into the blood or excreted in feces. The layer of tissue responsible for both absorbing nutrients and protecting against invaders is the epithelium. Our guts are a dynamic and complex environment home to 100’s of different bacteria species totaling nearly 1 kg of mass (Savage, 1977). In fact, there are more bacterial cells in the gut then tissue cells in the entire body (Bengmark, 1998).
The notion of good vs. bad bacteria in the gut was pioneered in the turn of the 20th century by Metchnikoff, a Russian scientist. Today we know that certain bacteria are harmful to humans by either causing infection and inflammation, or producing carcinogens and toxins; on the other hand, other strains of bacteria are beneficial (commensal microbiota). Commonly recognized and studied beneficial bacterium generas include Lactobacillus, Bifidobacterium, and Enterococcus; however, every individual distinct gut microbial composition. This composition fluctuates based upon nutritional, environmental, and psychological factors and affects the homeostasis of the individual (Guarner & Malagelada, 2003).
Beneficial bacteria and humans co-exist in a symbiotic relationship similar to the plovers that clean out the teeth of crocodiles. To better understand the potential benefits to probiotic supplementation, the specific metabolic, trophic, immunologic, and protective functions of commensal microbiota in the human gut will be discussed.
Metabolic
Transit time of foodstuff in the large intestine (colon) slows down significantly. It is due to this slow transit and elongated residency that bacteria are able to exert their positive or negative effects. One of the principal functions of commensal microbiota is the degradation of dietary or endogenously produced substrates. Bacteria that breakdown undigested carbohydrates and endogenously produced carbohydrates (such as glycoproteins in mucus) produce short chain fatty acids (SCFA) and anions. SCFA’s acidify the colon, prevent pathogenic bacteria growth, and influence intestinal motility. The SCFAs produced are rapidly absorbed, thereby increasing water and salt abosrbed, and are metabolized by the intestinal epithelia and the liver (Wong et al., 2006). SCFAs, specifically acetate, is a possible gluceogenic substrate and may reduce cholesterol by inhibiting the rate limiting enzyme in cholesterol synthesis, HMG-CoA (Roberfroid et al., 2010).
While SCFAs produced are beneficial, the putrefaction of amino acids produces potentially toxic sucstances such as ammonia, amines, phenols and indols. When toxin production exceeds liver clearance circulatory and liver pathologies may ensue (Picard et al., 2005). Beneficial bacteria, however, maintain colonic pH, enhance end-product degradation, produce sulfurous and ammonia reduction equivalents, reduce carcinotoxic compounds, suppress putrefactive microbes, and produce definsins against harmful competitors (Ouwehand et al., 2009; Roberfroid et al., 2010). Imbalances between beneficial and toxin producing bacterium commonly result in digestive disorders such as inflammatory bowel syndrome, sulfurous gas, diarrhea, bloating, cramping, and heart burn (Hedin et al. 2007).
Commensal microbiota also have profound influences on nutrient absorption. The absorption of ions such as calcium, phosphorus, magnesium, and iron are significantly improved through carbohydrate fermentation and the resultant production of SCFA (Younes et al., 2001). Commensal microbiota in the colon are able to synthesize vitamins a wide range of B vitamins including thamine, riboflavin, niacin, pantothenic acid, B6, biotin, folic acid, and B12 as well as vitamin K, and antioxidants (Conly et al., 1994; Hill, 1997; Pompei et al., 2007). In fact, lactobacillus reuteri supplementation was recently shown to restore the effects vitamin B12 deficiency (Molina et al., 2009). Thus, taken collectively, an ideal balance between beneficial and toxin producing bacteria in the colon is necessary to maintain digestive health, optimize nutrient absorption, and reduce digestive disorders (Figure 1).
Figure 1: A schematic representation of the symbiotic relationship between humans and resident commensal microbiota. Solid lines represent high-investigated mechanisms while dotted lines represent proposed interactions.
Adapted from Helen (2006).
Trophic
SCFAs produced by commensal microbiota stimulate epithelial proliferation and differentiation. Crypt cell production rates and cell density in crypts are reduced in the colons of bacteria free rats compared to rats colonized with microflora (Alam et al., 1994). The differentiation of epithelial cells is significantly modulated via interactions with resident microbes (Hooper et al., 2001). Lactobacillus, Bifidobacterium and S. thermophilus have all been shown to reduce colonic DNA damage after exposure to genotoxins. Supplementation with Bifidobacterium longus was demonstrated a 100% colonic tumor inhibition, significant reduction in the toxin-induced colonic aberrant crypt foci, and a significant decrease in colon tumor incidence, multiplicity and volume (Singh et al., 1997). Therefore, optimal levels of beneficial bacteria in the colon may play a role in the prevention of some pathological states such as chronic ulcerative colitis and colonic carcinogenesis (Guarner & Malagelada, 2003).
Immunologic
The intestinal mucosa represents the most physiologically significant interaction between the external and internal environment; thus, it is not surprising that the greatest mass of lymph tissue (gut-associated lymphatic tissue – GALT) and immunocompetent cells exist in the human body. Microbes, both pathogenic and beneficial, exert profound effects on the systemic and local immune system. In particular, commensal microbiota have been implicated in oral tolerance to pathogens. For example, mice bred in germ-free environments display a two day peripheral tolerance (immunity) to antigen ingestion compared to a two month tolerance to the same antigen in gnotobiotic mice. Interestingly, the abnormality was corrected with reconstitution of conventional colonic microbiota in young mice (Moreau & Gaboriau, 1996; Tanaka & Ishikawa, 2004).
Protective – The Barrier Effect
In order to influence the internal environment (your health), ingested pathogens must interact with or cross the intestinal mucosa. The intestinal mucosa represents a barrier between the blood and the digestive system, and therefore plays a critical role in immunity. Commensal microbiota play a critical role in the resistance to colonization and invasion of pathogenic microbes. Thus, an optimal equilibrium of resident colonic microbiota is essential to stability within the same individual under dynamic conditions (Guarner & Malagelada, 2003).
Mice bred in germ-free environments show a significantly greater susceptibility to infection compared to gnotobiotic mice (Taguchi et al., 2002). Liévin et al. (2000) suggested three mechanisms where by resident commensal microbiota may protect the individual from pathogens: antibacterial secretions, inhibition of pathogen adhesion, and immune stimulation. Indeed, Liévin et al.’s suggestions have been found to operate in animal models. Several strains of Bifidobacterium have been found to produce antimicrobial bacteriocins that inhibit pathogenic bacteria growth and B. longum has been shown inhibit the growth of toxic E. coli (Fujiwara et al., 1997; Liévin et al, 2000). In addition, resident microbes lower gut pH which decreases pathogenic microbe growth. Bernet et al. (1994) showed that Bifidobacterium both competes with pathogenic bacteria for binding sites and prevent epithelial crossing by pathogens. Hooper et al. (1999) found that Lactobacillus not only competes with pathogenic bacteria for nutrients, but also communicates energy needs with the host in order to reduce energy available to pathogenic microbes. Finally, Bifidobacterium was shown to increase immunological and defensive functions via enhanced pathogenic specific IgA-antibody production (Yasui et al., 1995).
Lifestyles and Pathologies Associated with
Colonic Microbiota Imbalance
The impact of resident and pathogenic colonic microbes does not just affect one’s digestion but exert profound effects on the overall health of the individual. Environmental factors such as stress or polution can influence the colonic microbial colony. Symptoms such as gas, bloating, constipation and heart burn are often due to an imbalance in resident colonic microbiota and can cause systemic disorders and disease. To illustrate the potential health and performance promoting effects of probiotic supplementation, the environmental changes that lead to and the systemic disturbances associated with resident commensal microbiota deficiency must first be discussed.
Environmental Influences
Growth of microbes in the digestive tract begins immediately after birth and colonization occurs within a few days. The type of birth (canal vs. caesarian), the diet of the new born (breast milk vs. formula), and even the hospital ward the infant was born in have an impact on the colonic microbial makeup. Studies show that in breast milk fed newborns the ratio of beneficial (anaerobic) to potentially harmful (aerobic) bacteria is between 100-1000:1 (Simon & Gorbach, 1984); however, environmental changes later in life can significantly impact the composition of colonic microbiota, and thus have profound effects on the individual’s health.
- 1. Nutrition
Commensal microbiota are fueled primarily through indigestible carbohydrate sources; however, not all fiber is fermented by residential microbes. Most insoluble fibers, such as the cellulose found in leafy vegetables and whole grains, are not fermented. Oligosaccharides, such as inulin, poly-fructose molecules, and poly-dextrose molecules are fermented by commensal microbiota and can be found in a variety of plants; however, increased consumption is often not enough to sufficiently stimulate healthy bacteria, offset prior damage, and positively influence the individual (Ling et al., 1994).
- 2. Stress
Psychological stress is an everyday part of the industrialized western world and has been shown to impair cellular immunity (Paik et al., 2000). Psychological stress, especially early in life, has been shown to alter colonic microbial biology (O’Mahoney et al., 2009) and contributes toward gut dysfunction (Molina et al., 2011). Due to resident commensal microbiota suppression during conditions of psychological stress, bacterial adhesion, translocation, and invasion is enhanced (Zareie et al., 2006), and may contribute significantly toward increased infections and chronic disease. In addition, adhesion of pathogenic microbes results in the release of inflammatory substances into the blood, which have been implicated in several conditions (discussed later).
- 3. Alcohol
While the majority consumed alcohol is absorbed in the small intestine, several consequences occur downstream in the colon. It is widely suggested that alcohol “kills” commensal microbiota; however, more research is needed before definite conclusions can be drawn. Alcohol consumption does, however, disrupt several metabolic processes that are intimately connected with resident colonic microbiota.
First, alcohol consumption damages the digestive mucosa (Kvietys et al., 1990). Second, and perhaps most important, alcohol consumption compromises gut-barrier function and increases the translocation of microbe derived lipopolysaccharide (LPS). LPS is transported via the portal vein where it is detoxified by the liver, resulting in hepatic inflammation. LPS is also aB. subtilisorbed by the lymphatic system and emptied directly into circulation, causing a release of inflammatory cytokines, and resulting in amplified inflammation and damage in the brain, liver, lung and heart (Wang et al., 2010). Finally, alcohol consumption significantly reduces thiamin (vitamin B1) and biotin absorption (Subramanya et al., 2010).
- 4. Antibiotics
Systemic infections occur when bacteria cross the intestinal epithelia and enter the blood, often the result of a weak or damaged intestinal mucosa, or colonization of pathogenic bacteria in the gut. Antibiotics are one of the most prescribed and frequently over-prescribed medicines in the industrialized world and are indicated to treat bacterial infections by either killing, injury, or inhibiting bacterial multiplication. While antibiotics can be effective in treating bacterial infections, the use of antibiotics also destroys resident commensal microbiota colonies, and may trigger intestinal inflammation and drive extra-intestinal immune-mediated diseases (Croswell et al., 2009; Reikvam et al., 2011; Willing et al., 2011). Because antibiotics are mis- or over-prescribed, pathogenic bacteria often become antibiotic resistance furthering the imbalance between resident beneficial and pathogenic microbes, and increasing the risk of infection (Croswell et al., 2009).
- 5. Pollutants
Air quality is most commonally associated with changes in respiratory anatomy, specifically higher incidences of lung infection and cancer in poor atmospheric conditions (Pope III et al., 2002). Recently, the affects of air pollution on the colonic microbial have been investigated. Kramar (2002) reported that the technogenic burden on air quality in an industrial city was associated with a 65-75% incidence of atypical variance in microflora. Leshchuk et al. (2011) reported individuals living in cities with high levels of air pollution showed bifidobacterium and lactobacillus deficiency in conjunction with elevated pathogenic bacterias. Finally, Savilov and Shcherbakova (2003) found that technogenic ambient air pollution significantly impacted the incidence and severity of S. sonnei dysentery.
Systemic Disturbances
Lifestyle and environment can profoundly affect the balance and mass of beneficial resident colon microbes. Disturbances in colonic microbiota composition can have local and systemic effects on the individual. The following sections will highlight the role colonic microbes play in various disorders to allude to the potential treatment effects of probiotic supplementation.
- 1. Diarrhea and Inflammatory Bowel Disease
Inflammatory bowel diseases (IBD) such as Crohn’s disease and ulcerative colitis present with the following: symptoms abdominal pain, vomiting, diarrhea, rectal bleeding, and severe cramps. IBD increases the risk of colorectal cancer and may become fatal if progression to toxic megacolon or intestinal perforation. Patients with IBD have higher amounts of pathogenic bacteria both attached to and within intestinal epithelial cells (Swidsinski et al., 2002). According to Pirzer et al. (1991), IBD is associated with over active T-cells responding to certain colonic microbes by releasing inflammatory cytokines and thus launching the inflammatory cascade via IgG. Because local tolerance mechanisms are abrogated, IgG further damages the intestinal muscosa, increasing bacterial adhesion, stimulating another immune response, and furthering the continuation of the inflammation cycle. Finally, IBD is associated with significant, though potentially reversible, imbalances and deficiencies in commensal microbiota (Ott et al., 2004; Stephani et al., 2011).
- 2. Systemic inflammation
Systemic inflammation is a common marker in several non-communicable diseases including diabetes and atherosclerosis. Imbalances in resident colonic microbiota and increased pathogenic bacteria adhesion result in increased bowel inflammation, and when combined with a weakened epithelial barrier, the translocation of inflammatory cytokines into the blood may result in systemic inflammation. Cani et al. (2008) found that concentrations of inflammatory cytokines (TNF-α, IL-1, IL-6, plasminogen activator inhibitor) in the blood are chronically elevated as a result of increased translocated microbe derived LPS translocation According to Tlaskalová-Hogenová et al. (2004), disturbances in commensal microbiota may play a role in autoimmune and chronic metabolic diseases. Indeed, chronic heart failure is associated with a weakened epithelial barrier, disturbed intestinal microcirculation, increased pathogenic bacteria adhesion, and increase in circulating gut derived inflammatory cytokines (Sandec et al., 2008, 2009). Therefore, maintaining an optimal ratio of functional resident commensal microbiota may be essential for controlling inflammatory “flare-ups” and promoting health.
- 3. Weight gain
Nearly 2 out of 3 adults in the United States are overweight or obese. While over consumption and under-activity are certainly causing factors of weight gain, Scarpellini et al. (2010) suggests that alterations in the colonic microbial environment and an increase in putative microbes may play also play a role in energy balance control. DiBaise et al. (2008) reported deifferences in the ratio of firmicutes to bacteroidetes in lean versus obese populations: obese subjects had significantly more firmicutes and less bacteriodetes compared to lean subjects. These metabolic changes, however, may be completely reversible with a reduction in caloric consumption and selective increases in specific gut microbes (Cani et al. 2007).
Resident commensal microbiota have been shown to activate fasting induced adipose factor (FIAF), which in turn inhibits adipose lipoprotein lipase (LPLa). Abnormal bacteria, however, have been shown to reduce FIAF. As a consequence, fatty acids are released from VLDL and chylomicrons into adipose capillaries, and subsequently taken up and stored as triglycerides in adipose tissue (Figure 2). Abnormal bacteria also have been shown to break down normally non-digestible polysaccharides (insoluable fiber) in monosaccharide’s, which are absorbed in the colon and transported to the liver via the portal vein. As a result, lipogenesis is activated via the hepatic carbohydrate responsive element binding protein (ChREBP), and non-alcoholic fatty liver syndrome and dyslipidemia may ensue (Cani & Delzenne, 2009). In addition, abnormal bacteria in the colon may reduce AMPK enzyme expression in liver and skeletal muscles, thus reducing fatty acid oxidation (Scapellini et al., 2010). While further research is currently underway to investigate the causal relationship between abnormal gut bacteria and obesity, maintaining a balanced colonic microbial ecosystem may be essential to body weight management.
Figure 2: Flow chart of mechanisms by which an imbalance in colonic microbiota influences changes in body composition.
- 4. Carcinogen Activation and Colon Cancer
While genetic makeup contributes significantly toward the development of colorectal cancer, environmental factors such as nutrition and carcinogen consumption have also been identified. A relationship exists between the increased risk of colorectal cancer and the consumption of red meats and high fats compared with that of high plant and fish sources (Bingham, 1999). Recently, however, the relationship between red meat consumption and colorectal cancer has been suggested to be mediated by alterations in colonic microbial composition and metabolism. The cooking of meat produces heterocyclic aromatic amines, a known initiator and promoter of colon cancer. In addition, healthy subjects who consume high quantities of red meat and void of vegetables excrete an increase in fecal genotoxic substances. Interestingly, differences in the response and metabolism to genotoxic substances have been found in varying species of colonic microbes: bacteroides and clostridium genera were shown to increase damage epithelial DNA whereas lactobacillus and bifidobacteria were shown to prevent tumor growth (Horie et al., 1999). While the exact mechanisms whereby variances in colonic bacteria may influence colorectal cancer is still under investigation, maintenance of the composition and activity of commensal microbiota may be a major environmental factor in modifying the risk of proliferative colonic diseases (Pagnini et al., 2008).
- Depression
Human subjects with depression have been shown to have abnormal respiratory hydrogen excretion following the consumption of fructose and other sugars. The elimination of fructose from the diet resulted in an improvement in depression symptoms (Ledochowski et al., 2000). Mal-absorption of fructose is accompanied by a reduction in plasma tryptophan (which is used in the synthesis of serotonin), in addition, fructose mal-absorption provides substrate for rapid fermentation and thus disturbs colonic microbiota composition (Ledochowski et al., 2001). Therefore, Collins and Bercik (2009) have suggested that relationship between carbohydrate mal-absorption and depression symptoms may be a result of bacterial interference with tryptophan metabolism. O’Mahoney et al. (2009) suggests that an alteration in colonic microbiota may be a possible contributor of psychiatric distress (Figure 3).
Figure 3. The role of colonic microbiota in regulating behavior. The left depicts a balanced colon with an optimal composition and functioning of commensal microbiota. The right demonstrates intestinal inflammation and depicts the cyclical relationship between emotional stress, microbial dysfunction, inflammation, and psychiatric distress.
Adapted from Collins & Bercik, 2009
Interactions between the gut and brain (GBA) are made via the immune system (Figure 4). A top-down approach examining the effect of variations in stress and anxiety on the gut has shown that the brain can have significant effects on the digestive system (Aziz & Thompson, 1998). More recently, however, a bottom-up approach has been used to study the effects of the gut (specifically that of the colonic microbiota) on the brain. Receptors in the GI tract are sensitive to inflammatory cytokines, impinge on vagal nerve afferents, and cause anxiety-behavior in rats (Wang et al., 2002). Preliminary research has shown no differences exist in stress hormone concentration between germ-free mice and gnotobiotic mice at rest; however, when exposed to a restraint stress, germ-free mice exhibit an exaggerated increase in corticosterone and adrenocorticotrophic hormone levels (Sudo et al, 2004). Deficits in learning have also been found in germ free mice under both stressed and stress-free conditions (Neufeld et al., 2008). The deficit in learning may have been mediated by a reduction in brain-derived neurotrophic factor, a major regulator of mood and cognitive function, which was observed in the cortex and hippocampus in the germ free mice. Interestingly, colonization of the gut with resident microbiota and the resultant restoration of the immune system normalized the stress response in adolescent mice (Sudo et al., 2004). Since gut inflammation via an imbalance in colonic microbiota may be a major contributor toward psychiatric distress (O’Mahoney et al., 2009; O’Malley et al., 2011), optimizing the mass and composition commensal microbiota may maintain cognitive function and mood during times of increased stress.
Figure 4. The integration of the gut-brain axis and microbiota. On the left is the the pathway whereby the brain influences colonic microbiota. The right is the mechanisms whereby colonic microbiota influence the brain.
Adapted from Collins & Bercik, 2009
The Priobiotic Concept
By now it should now be clear that a direct link exists between disorders of the gut and the entire body. Metabolic disorders previously thought to be a product of genetics, nutrition and activity such as dyslipidemia, weight gain, and colon cancer are now known to be highly influenced by the composition and function of colonic microbes. It is also now understood that colonic inflammation and malfunction effects more than the digestive system; colonic inflammation can trigger systemic inflammation which can negatively affect rhumatic diseases, heart conditions, diabetes, and athersclerotic disease. In addition, significant relationships exist between colonic disturbances and the CNS; colonic inflammation and malfunction can affect learning memory and cognition, and has been linked to emotional distress such as depression, anxiety, fear, and chronic fatigue (Mayer, 2011).
Figure 5. Schematic of gut to brain signaling influenced by colonic microbiota. Disturbances in colonic microbiota result in homeostatic perturbations (green); whereas functional commensal microbiota maintain gut homeostasis (Blue). Colonic disturbances are sensed via the anterior insula (aINS) where they manifest as gut discomfort. The aINS stimulates limbic structures that report to higher brain centers resulting in the negative modulation of mood and cognition.
(AMYG – amygdale; CCK – cholecystokinin; HIPP – hippocampus; NPY – neuropeptide Y; OFC – orbitofrontal cortex; SCSA – short chain fatty acid)
Adapted from Mayer, 2011
According to World Health Organization (Araya et al., 2001), probiotics are “live micro-organisms which confer a health benefit on the host when administered in adequate amounts.” To improve upon the information in this paper, the definition by Aray et al. (2001) will be expanded to include the caveat that: “When consumed, probiotic organisms must be able to survive passage through the digestive tract and have the capability to proliferate in the colon.” (Araya et al., 2002). Therefore, for a probiotic supplement to be effective, it must either be resistant to gastic juices and bile, or in a vehicle capable of delivering the micro-organism to the colon. These are most commonly anaerobic gram-positive bacteria belonging to the Lactobacillus and Bifidobacterium genera; however, other non-pathogenic strains of lactic acid bacterium such as Streptococcus and Enterococcus have also been investigated.
Probiotics are safe for human consumption, pose no health risks, and the beneficial, treatment, and preventative effects have been studied in both humans and animal (Borriello et al., 2003). Probiotics are able to improve both the development and the stability of the commensal microbiota and exert positive changes by inhibiting pathogen colonization and adhesion, strengthening the mucosa barrier by positively influencing intestinal epithelia, improving colonic transit, metabolizing carcinogens, and reducing colonic inflammation. Researchers (An et al., 2011; Araya et al., 2001; Picard et al., 2005; Tlaskalová et al., 2004) have suggested that probiotic supplementation may be beneficial in both health and disease by:
- Improvement and/or stabilization of gut microbiota composition
- Improvement of intestinal functions (stool bulking, stool regularity, stool consistency)
- Increase in mineral absorption and improvement of bone health (bone Ca content, bone mineral density)
- Modulation of gastro-intestinal peptides production, energy metabolism and satiety
- Initiation (after birth) and regulation/modulation of immune functions
- Improvement of intestinal barrier functions, reduction of metabolic endotoxemia
- Reduction of risk of intestinal infections and tentatively
- Reduction of risk of obesity, type 2 diabetes, metabolic syndrome, etc.
- Reduction of risk and/or improvement in the management of intestinal and systemic inflammation
- Prevention and therapy for ischemic heart disease
- Serum cholesterol reduction
- Reduction in vaginal bacterial infections
- Reduced of risk of colon cancer
- Reduction in respiratory infections
- Regulation of adipose tissue deposition
- Improvement in stress response, anxiety, and emotional stability
Research Investigating the Health and Performance Benefitsof the Probiotic Strains in Gut Health
The purpose of this paper is two-fold: first, to educate the reader regarding the intimate relationship and important functions of colonic bacteria with the individual; and second, to educate the consumer regarding use and effectiveness of probiotics. After reviewing the research regarding specific strains of probiotic bacteria, investigating the ingredients of popular probiotic products on the market, and several personal and client based trials with various probiotic products, I now feel comfortable making product suggestions to consumers.
The following sections will therefore review research investigating the bacteria species found in Gut Health, my personal recommendation to consumers wishing to benefit from probiotic supplementation.
Bacillus subtilis
Prior to the introduction (and over-prescription) of antibiotics into western medicine, B. subtilis was used to treat GI and urinary tract diseases. To be an effective probiotic, the strain must first be able to survive the GI tract and then grow in the colon: B. subtilis fulfills this role (Leser et al., 2008). B. subtilis exerts beneficial effects by positively influencing infection resistance, immunity, inflammation, and digestive health.
In animal models, B. subtilis lowered morbidity and mortality due to E. coli infection, reduced susceptibility to C. rodentium and salmonella infection, and improved mucosal barrier function (Alexopoulos et al., 2004; D’Arienzo et al., 2006; Fujiya et al., 2007; Knap et al., 2011). B. subtilis positively modulated inflammation via reduced colonic inflammatory cytokines (TNF-alpha, 1L-1beta, IL-6 and IFN-gamma) and increase anti-inflammatory cytokine secretion (IL-I0 and TGF-beta) (Selvam et al., 2009). B. subtilis has also been shown to protect mucosal epithelia against inflammatory and oxidative damage by activating cyto-protective heat shock proteins (Fujiya et al., 2007; Williams, 2007). A novel, yet very appealing finding to probiotic supplementation may be that B. subtilis can improve body composition. In growing pigs, addition of B. subtilis to a nutrient dense enhanced lean body mass gain, reduced fat mass gain, and improved nutrient assimilation in a dose dependent manner (Alexopoulos et al., 2004; Meng et al., 2004).
Bacillus coagulans
B. coagulans is a spore producing bacterium. When exposed to low pH, such as in the stomach, these spores are activated, absorb water, and then germinate (Sander et al., 2003). B. coagulans proliferates and colonized in the colon and therefore is capable of exerting effects on the individual. B. coagulans has been used to treat IBD and Crohn’s disease, increase influenza immunity, improve autoimmune diseases, and reduce inflammation.
The beneficial effects of B. coagulans are most likely exerted via reductions in pathogenic bacteria colonization and adhesion. B. coagulans has been shown to exhibit anti-microbial activity in the face of pathogenic bacteria, preventing disturbances in the colonic microbiota ecosystem (Honda et al., 2011). By competing with pathogenic bacteria, B. coagulans is able to maintain colonic homeostasis. In human studies, B. coagulans has been demonstrated to reduce bloating and abdominal discomfort in patients with IBD (Hun, 2009). In humans without IBD, B. coagulans has been shown reduce post-prandial gas and bloating, and improve the quality of life and reduce GI symptoms (Kalman et al., 2009). B. coagulans also exerts positive metabolic effects. In human trials, B. coagulans was shown to significantly lower total cholesterol and LDL cholesterol while slightly increasing HDL cholesterol (Mohan et al., 1990).
B. coagulans has been used to treat rheumatoid arthritis. In a controlled clinical trial, B. coagulans improved walking ability, sit and reach, and daily activity participation without any adverse effects (Mandel et al., 2010). B. coagulans reduces inflammation by introducing cell membrane components and metabolites that modulate cytokine production. In particular, B. coagulans reduces inflammatory cytokines TNF-alpha and IL-2, and increases production of anti-inflammatory cytokines IL-10 (Jensen et al., 2010). B. coagulans supplementation also reduces C-reactive protein concentrations, a significant marker in the risk of cardiovascular disease (Park et al., 2010). In addition, B. coagulans positively impacts the immunity of the individual. B. coagulans has been shown to enhance T-cell response influenza and adenovirus, and has also been shown to enhance the immune response to respiratory viral tract infections (Baron, 2009; Kimmel et al., 2010).
Lactobacillus plantarum
L. planatarum is a lactic acid producing bacteria commonly found in many fermented foods such as pickles, certain cheeses, and brined fish. L. planatarum is a resident salivary and colonic microbe. More so, L. planatarum is able to survive passage through the GI of both mouse and human, and readily colonize in the colon (Bron et al., 2004). Supplemental L. planatarum has been to treat IBD and Crohn’s disease, enhance immunity, reduce inflammation, and has recently shown promising metabolic effects.
Elderly individuals may especially benefit from L. planatarum supplementation. Mañé et al. (2011) supplemented institutionalized elderly subjects in a nursing home with a high dose of L. planatarum, a low dose of L. planatarum, or a placebo for 12 weeks. At the conclusion of the supplementation period a dose dependent reduction in incidence of infection was found. TGF-β, a potential colon cancer promoting cytokine, also decreased in both the supplemented groups, and these changes were still present 12 weeks following L. planatarum discontinuation. Additionally, Bosch-Gallego et al. (2011) recently reported that L. planatarum supplementation improved intestinal transit and nutrient status, and improved the over-all quality of life in elderly patients. Therefore, L. planatarum may be a valuable treatment in preventing infections and improving life in older populations.
L. planatarum also increases immunity in young, healthy subjects. L. planatarum adheres to colonic epithelia and prevents the pathogenic adherence and colonization via competition, exclusion, and displacement. In addition L. planatarum secretes antibacterial factors such as bacteriocins, lactic acid and exopolysaccharides (Satish Kumar et al., 2011). L. planatarum has the highest level of adherence to colonic epithelia and has been shown to defend against rotavirus and gastroenteritis viral infections (Maragkoudakis et al., 2010). In addition, L. planatarum may improve food related allergic reactions, such as soy allergies (Frias et al., 2008).
L. planatarum may help treat or alleviate the symptoms of many inflammatory systemic and GI diseases. In animal models, L. planatarum has been shown to reduce NF-kappaβ binding and inhibit proteosome activity in mice, increase nitric oxide production under conditions of inflammation, reduce inflammatory cytokine (TNF-α and IL-1β) production of monocytes, and decrease LPS stimulated TNF-α production (Kang et al., 2011; Kim et al., 2008, 2011; Petrof et al., 2009). Due to reduced inflammation, L. planatarum may successful treat IBD, colitis, and reduce gastric colorectal distension and abdominal pain (Dunker et al., 2011; Niedzielin et al., 2001).
Because L. planatarum positively influences cardiovascular disease, L. planatarum maybe used to help treat and reduce the risk of cardiovascular disease. L. planatarum has been shown to reduce C-reactive protein, a marker of cardiovascular disease (Bosch-Gallego et al., 2011). In smokers, L. planatarum reduced blood pressure, clotting factors (fibrinogen), inflammatory cytokines, and monocyte adhesion to vascular endothelial cells (Naruszewicz et al., 2002). In an animal model, L. planatarum was shown to reduce hypertension via angiotensin-converting enzyme 1 inhibition (Liu et al., 2011). In moderately hyperchosteremic humans, L. planatarum has been shown to reduce fibrinogen and LDL cholesterol (Bukoska et al., 1998). L. planatarum increases bile acid deconjugation (Jones et al., 2004). Because bile synthesis requires cholesterol, one mechanism by which L. planatarum improves cholesterol metabolism is via bile acid excretion (Juen et al., 2010). Therefore, individuals with risk factors for cardiovascular disease may benefit greatly from L. planatarum supplementation.
The metabolic and potential body composition enhancing effects of L. planatarum supplementation are also quite intriguing. First, L. planatarum has been shown to increase amino acid absorption via enhanced oligopeptide transporter 1 activity (Chem et al., 2010). Second, L. planatarum reduces adipose cell size and reduces adiponectin in mice fed a high fat diet (Takemura et al., 2010). L. planatarum has also been shown to inhibit adipogenesis in vitro via reduced fatty acid synthase and adipocyte-fatty acid binding protein expression (Park et al., 2011). Thus, L. planatarum smay be a valuable supplement in individuals wishing to improve body composition because of its positive nutrient partitioning effects.
Streptococcus thermophilus
S. thermophilus is one of the most important bacteria in the food industry and used to produce yogurt, cooked cheeses, and soft cheeses. S. thermophilus has been shown to survive in a 0.4% oxgall concentration (highest bile concentration of duodenum) and a pH of 2 for 3 hours, demonstrates a high adherence to the intestinal mucosa, and is highly antibiotic resistant (Khali, 2009). S. thermophilus is a viable bacterium that enhances the composition of colonic microbiota (Liu et al., 2010), and therefore has the ability to exert probiotic effects such as inflammatory reductions, inhibition of carcinogen production, enhanced immunity, and as a possible treatment in chemo-therapy induced intestinal mucositis.
By reducing cytokines that play significant roles in inflammatory disease, S. thermophilus has been suggested a possible therapy in intestinal and systemic disease. S. thermophilus has been shown to suppress IL-17 expression and production by splenocytes (Oquita et al., 2011). S. thermophilus has been used to treat chronic gastritis by reducing IFN-ƴ and TNF-α, and increasing the thickness of the gastric mucus gel layer (Rodriguez et al., 2010).
S. thermophilus enhances the immune system via diverse set of mechanisms. First, S. thermophilus is able to transfer genes (CRISPR3/Cas) directly to pathogenic bacteria, such as E. coli (Sapranauskas et al., 2011). CRISPR3/Cas cleaves bacteriophage DNA, and thus provides protection against viral gene transfer and bacterial infection (Barrangou et al., 2007). Second, S. thermophilus secretes antimicrobial bacteriocins that directly prevent the adhesion and colonization of pathogenic bacteria (Gilbreth & Somkuti, 2005). Finally, in a mouse model, S. thermophilus stimulated T-cells when presented with pathogens (Shimosato et al, 2009). Because S. thermophilus is able to positively influence the colonic microbiota, S. thermophilus may be an important supplement following the use of antibiotics, during times of increased mental stress, and prior to/following travel to polluted cities.
Lactobacillus casei
In vitro simulations of the stomach and small intestine show a high survival rate for L. casei (Lo Curto et al., 2011). Fecal examination has demonstrated that L. casei does indeed survive the human GI tract (Yuki et al., 1999). In addition, L. casei is able to adhere and colonize in the human colon following regular consumption, and has also been shown to positively influence the resident colonic microbiota (Tuohy et al., 2007). Additionally, L. casei supplementation in infants has been shown to promote a stable colonic microbial environment and improve tolerance to allergic disease (Cox et al., 2010).
Individuals suffering from sulfurous gas and bloating may benefit greatly from L. casei supplementation. L. casei has been shown to metabolize volatile sulfer and ammonia in vitro (Naidu et al., 2002). Because an accumulation of sulfide and ammonia can be carcinogenic and toxic to the liver, L. casei may therefore be a valuable supplement to individuals consuming high protein diets as well.
L. casei has been suggested as a possible treatment in dyslipidemia. Usman & Hosono (2000) reported that hypercholesterolemic mice fed L. casei exhibited lower total serum, LDL, and triglycerides due to suppression of bile absoprtion into the enterohepatic circulation and an increase in acidic steroid excretion. Mice fed a combination of L. casei and oligosaccharide (a non-digestible polysaccharide) exhibited a significant decrease in serum total and LDL cholesterol, as well as an increase in HDL cholesterol. In addition, an increase in lactic acid due to oligosaccharide fermentation reduced pathogenic bacteria in the colon (Liong & Shah, 2006).
Inflammation reductions have also been reported with L. casei supplementation. Supplementation of L. casei with type II collagen and glucosamine significantly reduced inflammation, pain, cartilage destruction, and lymphocyte infiltration in a mouse model of osteoarthritis. The protocol reduced inflammatory cytokines (IL-1β, IL-2, IL-6, IL-12, IL-17, TNF-α, IFN-γ) and cartilage tissue degraders (MMP1, MMP3, MMP13) while increasing anti-inflammatory cytokines (IL-4, IL-10). In addition, expression of MMP inhibitors and type II collagen in chondrocytes were also increased (So et al., 2011). Thus, L. casei may not only be able to treat osteoarthritis by reducing inflammation, but may also contribute to improved recovery time from cartilage injuries by increasing type II collagen expression in chondrocytes.
Summary
Humans and commensal bacteria exist in a symbiotic relationship. Commensal bacteria contribute to health and well being by defending against disease, improving nutrient absorption, and maintaining metabolic processes. The modern western world poses many stressors that damage the harmony of the colonic microbial environment including stress, poor nutrition, pollutants such as air pollution and food additives, alcohol consumption, and antibiotics. These stressors result in an imbalance of the colonic microbiota and can result in digestive diseases such as inflammatory bowel disease and chronic diarrhea; gastrointestinal and colorectal cancers; increased adiposity; systemic inflammatory diseases such atherosclerosis and cardiovascular disease; increased pain and symptoms of autoimmune diseases such as rheumatoid arthritis; and contribute to anxiety and depression.
The colonic microbiota, however, is a dynamic environment that may is enhanced with improved diet, life style, and probiotic supplementation. In particular, the bacterial strains in Gut Health demonstrate a high potential to treat a variety of diseases and improve health. Healthy individuals suffering from ailments such as gas, bloating, and abdominal discomfort can benefit greatly from Gut Health supplementation. The elderly may especially benefit from the improved intestinal transit and immunity effects of Gut Health. In addition, the probiotics in Gut Health have been shown to increase resistance against bacterial and viral infections, and therefore may especially useful to people who suffer from seasonal allergies, flu, are exposed to bacteria by eating meats or uncooked vegetables, and those who work around people with common colds, such as students, school teachers, nurses, and public servants.
Gut Health also appears to have the ability to improve symptoms and even treat individuals suffering from inflammatory diseases such as IBD, Crohn’s disease, gastritis, rheumatoid arthritis, and osteoarthritis by reducing inflammation. The probiotics in Gut Health have been shown to improve cardiovascular disease by reducing C-reactive protein, improving serum cholesterol (reducing total cholesterol, LDL, triglycerides and increasing HDL), and improving the symptoms of hypertension. Thus, Gut Health may be a great supplement to improve and reduce risk factors associated with cardiovascular disease.
Finally, Gut Health may improve athletic performance. The probiotics in Gut Health have been shown to increase protein and B-vitamin absorption, reduce adipose fatty acid synthesis and adipocyte size, and has even been shown to improve lean to fat mass ratio in pigs. Finally, athletes recovering from connective tissue based injuries such as tendonitis, sprains, strains, and cartilage damage may be able to improve recovery by supplementing with Gut Health. In particular, the L. casei strain in Gut Health increases collagen expression in chondrocytes and may reduce inflammation in connective tissue.
References
Acheson, D. W. K., & Luccioli, S. (2004). Microbial-gut interactions in health and disease. Mucosal immune responses. Best practice & research. Clinical gastroenterology, 18(2), 387-404. doi:10.1016/j.bpg.2003.11.002
Alam, M., Midtvedt, T., & Uribe, A. (1994). Differential cell kinetics in the ileum and colon of germfree rats. Scandinavian journal of gastroenterology, 29(5), 445-51. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8036460
Alexopoulos, C., Georgoulakis, I. E., Tzivara, A., Kyriakis, C. S., Govaris, A., & Kyriakis, S. C. (2004). Field evaluation of the effect of a probiotic-containing Bacillus licheniformis and Bacillus subtilis spores on the health status, performance, and carcass quality of grower and finisher pigs. Journal of veterinary medicine. A, Physiology, pathology, clinical medicine, 51(6), 306-12. doi:10.1111/j.1439-0442.2004.00637.x
An, H. M., Park, S. Y., Lee, D. K., Kim, J. R., Cha, M. K., Lee, S. W., Lim, H. T., et al. (2011). Antiobesity and lipid-lowering effects of Bifidobacterium spp. in high fat diet-induced obese rats. Lipids in health and disease, 10(1), 116. BioMed Central Ltd. doi:10.1186/1476-511X-10-116
Araya, M., Gopal, P., Lindgren, S. E., Lodi, R., Oliver, G., Saxelin, M., Servin, A., et al. (2001). Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics. Cordoba, Argentina: Food and Agricultural Organization of the United Nations. Retrieved from http://www.who.int/foodsafety/publications/fs_man
Araya, M., Morelli, L., Reid, G., Sanders, M., & Stanton, C. (2002). Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food (pp. 1-11). Ontario, Canada. Retrieved from http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:Guidelines+for+the+Evaluation+of+Probiotics+in+Food#1
Aziz, Q., & Thompson, D. G. (1998). Brain-gut axis in health and disease. Gastroenterology, 114(3), 559-78. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9496948
Bai, A.-P., Ouyang, Q., Xiao, X.-R., & Li, S.-F. (2006). Probiotics modulate inflammatory cytokine secretion from inflamed mucosa in active ulcerative colitis. International journal of clinical practice, 60(3), 284-8. doi:10.1111/j.1368-5031.2006.00833.x
Baron, M. (2009). A patented strain of Bacillus coagulans increased immune response to viral challenge. Postgraduate medicine, 121(2), 114-8. doi:10.3810/pgm.2009.03.1971
Barrangou, R., Fremaux, C., Deveau, H., Richards, M., Boyaval, P., Moineau, S., Romero, D. A., et al. (2007). CRISPR provides acquired resistance against viruses in prokaryotes. Science (New York, N.Y.), 315(5819), 1709-12. doi:10.1126/science.1138140
Bengmark, S. (1998). Ecological control of the gastrointestinal tract. The role of probiotic flora. Gut, 42(1), 2-7. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1726957&tool=pmcentrez&rendertype=abstract
Bernet, M. F., Brassart, D., Neeser, J. R., & Servin, A. L. (1994). Lactobacillus acidophilus LA 1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut, 35(4), 483-9. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1374796&tool=pmcentrez&rendertype=abstract
Bingham, S. A. (1999). High-meat diets and cancer risk. The Proceedings of the Nutrition Society, 58(2), 243-8. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10466162
Borriello, S. P., Hammes, W. P., Holzapfel, W., Marteau, P., Schrezenmeir, J, Vaara, M., & Valtonen, V. (2003). Safety of probiotics that contain lactobacilli or bifidobacteria. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 36(6), 775-80. doi:10.1086/368080
Bosch Gallego, M., Espadaler Mazo, J., Méndez Sánchez, M., Pérez Carre, M., Farrán Codina, A., Audivert Brugué, S., Bonachera Sierra, M. A., et al. (2011). [Consumption of the probiotic lactobacillus planctarum CECT 7315/7316 improves general health in the elderly subjects]. Nutrición hospitalaria : organo oficial de la Sociedad Española de Nutrición Parenteral y Enteral, 26(3), 642-5. doi:10.1590/S0212-16112011000300030
Bron, P. A., Grangette, C., Mercenier, A., de Vos, W. M., & Kleerebezem, M. (2004). Identification of Lactobacillus plantarum genes that are induced in the gastrointestinal tract of mice. Journal of bacteriology, 186(17), 5721-9. doi:10.1128/JB.186.17.5721-5729.2004
Bukowska, H, Pieczul-Mróz, J., Jastrzebska, M., Chełstowski, K., & Naruszewicz, M. (1998). Decrease in fibrinogen and LDL-cholesterol levels upon supplementation of diet with Lactobacillus plantarum in subjects with moderately elevated cholesterol. Atherosclerosis, 137(2), 437-8. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9622287
Cani, P D, Delzenne, N M, Amar, J., & Burcelin, R. (2008). Role of gut microflora in the development of obesity and insulin resistance following high-fat diet feeding. Pathologie-biologie, 56(5), 305-9. doi:10.1016/j.patbio.2007.09.008
Cani, P D, Neyrinck, A M, Fava, F., Knauf, C., Burcelin, R. G., Tuohy, K. M., Gibson, G R, et al. (2007). Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia, 50(11), 2374-83. doi:10.1007/s00125-007-0791-0
Cani, Patrice D, & Delzenne, Nathalie M. (2009). The role of the gut microbiota in energy metabolism and metabolic disease. Current pharmaceutical design, 15(13), 1546-58. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19442172
Chen, H.-Q., Shen, T.-Y., Zhou, Y.-K., Zhang, M., Chu, Z.-X., Hang, X.-M., & Qin, H.-L. (2010). Lactobacillus plantarum consumption increases PepT1-mediated amino acid absorption by enhancing protein kinase C activity in spontaneously colitic mice. The Journal of nutrition, 140(12), 2201-6. doi:10.3945/jn.110.123265
Collins, S. M., & Bercik, P. (2009). The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology, 136(6), 2003-14. AGA Institute American Gastroenterological Association. doi:10.1053/j.gastro.2009.01.075
Conly, J. M., Stein, K., Worobetz, L., & Rutledge-Harding, S. (1994). The contribution of vitamin K2 (menaquinones) produced by the intestinal microflora to human nutritional requirements for vitamin K. The American journal of gastroenterology, 89(6), 915-23. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8198105
Cox, M. J., Huang, Y. J., Fujimura, K. E., Liu, J. T., McKean, M., Boushey, H. A., Segal, M. R., et al. (2010). Lactobacillus casei abundance is associated with profound shifts in the infant gut microbiome. PloS one, 5(1), e8745. doi:10.1371/journal.pone.0008745
Croswell, A., Amir, E., Teggatz, P., Barman, M., & Salzman, N. H. (2009). Prolonged impact of antibiotics on intestinal microbial ecology and susceptibility to enteric Salmonella infection. Infection and immunity, 77(7), 2741-53. doi:10.1128/IAI.00006-09
Cunningham-Rundles, S. (2001). Nutrition and the mucosal immune system. Current opinion in gastroenterology, 17(2), 171-176. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11224675
Lo Curto, A., Pitino, I., Mandalari, G., Dainty, J. R., Faulks, R. M., & John Wickham, M. S. (2011). Survival of probiotic lactobacilli in the upper gastrointestinal tract using an in vitro gastric model of digestion. Food microbiology, 28(7), 1359-66. doi:10.1016/j.fm.2011.06.007
DiBaise, J. K., Zhang, H., Crowell, M. D., Krajmalnik-Brown, R., Decker, G. A., & Rittmann, B. E. (2008). Gut microbiota and its possible relationship with obesity. Mayo Clinic proceedings. Mayo Clinic, 83(4), 460-9. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/18380992
Duncker, S. C., Kamiya, T., Wang, L., Yang, P., & Bienenstock, John. (2011). Probiotic Lactobacillus reuteri Alleviates the Response to Gastric Distension in Rats. The Journal of nutrition, Epub. doi:10.3945/jn.110.136689
D’Arienzo, R., Maurano, F., Mazzarella, G., Luongo, D., Stefanile, R., Ricca, E., & Rossi, Mauro. (2006). Bacillus subtilis spores reduce susceptibility to Citrobacter rodentium-mediated enteropathy in a mouse model. Research in microbiology, 157(9), 891-7. doi:10.1016/j.resmic.2006.06.001
Eutamene, H., & Bueno, L. (2007). Role of probiotics in correcting abnormalities of colonic flora induced by stress. Gut, 56(11), 1495-7. doi:10.1136/gut.2007.124040
Everhart, J. E., & Ruhl, C. E. (2009). Burden of digestive diseases in the United States part I: overall and upper gastrointestinal diseases. Gastroenterology, 136(2), 376-86. doi:10.1053/j.gastro.2008.12.015
Frias, J., Song, Y. S., Martínez-Villaluenga, C., González de Mejia, E., & Vidal-Valverde, C. (2008). Immunoreactivity and amino acid content of fermented soybean products. Journal of agricultural and food chemistry, 56(1), 99-105. doi:10.1021/jf072177j
Fujiwara, S., Hashiba, H., Hirota, T., & Forstner, J. F. (1997). Proteinaceous factor(s) in culture supernatant fluids of bifidobacteria which prevents the binding of enterotoxigenic Escherichia coli to gangliotetraosylceramide. Applied and environmental microbiology, 63(2), 506-12. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=168341&tool=pmcentrez&rendertype=abstract
Fujiya, M., Musch, M. W., Nakagawa, Y., Hu, S., Alverdy, J., Kohgo, Y., Schneewind, O., et al. (2007). The Bacillus subtilis quorum-sensing molecule CSF contributes to intestinal homeostasis via OCTN2, a host cell membrane transporter. Cell host & microbe, 1(4), 299-308. doi:10.1016/j.chom.2007.05.004
Gilbreth, S. E., & Somkuti, G. A. (2005). Thermophilin 110: a bacteriocin of Streptococcus thermophilus ST110. Current microbiology, 51(3), 175-82. doi:10.1007/s00284-005-4540-7
Guarner, F., & Malagelada, J.-R. (2003). Gut flora in health and disease. Lancet, 361(9356), 512-9. doi:10.1016/S0140-6736(03)12489-0
Hedin, C., Whelan, K., & Lindsay, J. O. (2007). Evidence for the use of probiotics and prebiotics in inflammatory bowel disease: a review of clinical trials. The Proceedings of the Nutrition Society, 66(3), 307-15. doi:10.1017/S0029665107005563
Hill, M. J. (1997). Intestinal flora and endogenous vitamin synthesis. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation (ECP), 6 Suppl 1, S43-5. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9167138
Honda, H., Gibson, Glenn R, Farmer, Sean, Keller, David, & McCartney, Anne L. (2011). Use of a continuous culture fermentation system to investigate the effect of GanedenBC30 (Bacillus coagulans GBI-30, 6086) supplementation on pathogen survival in the human gut microbiota. Anaerobe, 17(1), 36-42. doi:10.1016/j.anaerobe.2010.12.006
Hooper, L. V., Wong, M. H., Thelin, A., Hansson, L., Falk, P. G., & Gordon, J. I. (2001). Molecular analysis of commensal host-microbial relationships in the intestine. Science (New York, N.Y.), 291(5505), 881-4. doi:10.1126/science.291.5505.881
Hooper, L. V., Xu, J., Falk, P. G., Midtvedt, T., & Gordon, J. I. (1999). A molecular sensor that allows a gut commensal to control its nutrient foundation in a competitive ecosystem. Proceedings of the National Academy of Sciences of the United States of America, 96(17), 9833-8. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=22296&tool=pmcentrez&rendertype=abstract
Horie, H., Kanazawa, K., Kobayashi, E., Okada, M., Fujimura, A., Yamagiwa, S., & Abo, T. (1999). Effects of intestinal bacteria on the development of colonic neoplasm II. Changes in the immunological environment. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation (ECP), 8(6), 533-7. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10643943
Hun, L. (2009). Bacillus coagulans significantly improved abdominal pain and bloating in patients with IBS. Postgraduate medicine, 121(2), 119-24. doi:10.3810/pgm.2009.03.1984
Irmler, S., Raboud, S., Beisert, B., Rauhut, D., & Berthoud, H. (2008). Cloning and characterization of two Lactobacillus casei genes encoding a cystathionine lyase. Applied and environmental microbiology, 74(1), 99-106. doi:10.1128/AEM.00745-07
Jensen, G. S., Benson, K. F., Carter, S. G., & Endres, J. R. (2010). GanedenBC30 cell wall and metabolites: anti-inflammatory and immune modulating effects in vitro. BMC immunology, 11, 15. doi:10.1186/1471-2172-11-15
Jeun, J., Kim, S., Cho, S.-Y., Jun, H.-J., Park, H.-J., Seo, J.-G., Chung, M.-J., et al. (2010). Hypocholesterolemic effects of Lactobacillus plantarum KCTC3928 by increased bile acid excretion in C57BL/6 mice. Nutrition (Burbank, Los Angeles County, Calif.), 26(3), 321-30. doi:10.1016/j.nut.2009.04.011
Jones, M. L., Chen, H., Ouyang, W., Metz, T., & Prakash, S. (2004). Microencapsulated Genetically Engineered Lactobacillus plantarum 80 (pCBH1) for Bile Acid Deconjugation and Its Implication in Lowering Cholesterol. Journal of biomedicine & biotechnology, 2004(1), 61-69. doi:10.1155/S1110724304307011
Kalman, D. S., Schwartz, H. I., Alvarez, P., Feldman, S., Pezzullo, J. C., & Krieger, D. R. (2009). A prospective, randomized, double-blind, placebo-controlled parallel-group dual site trial to evaluate the effects of a Bacillus coagulans-based product on functional intestinal gas symptoms. BMC gastroenterology, 9, 85. doi:10.1186/1471-230X-9-85
Kang, S.-S., Ryu, Y. H., Baik, J. E., Yun, C.-H., Lee, K., Chung, D. K., & Han, S. H. (2011). Lipoteichoic acid from Lactobacillus plantarum induces nitric oxide production in the presence of interferon-γ in murine macrophages. Molecular immunology, 48(15-16), 2170-7. doi:10.1016/j.molimm.2011.07.009
Khalil, R. (2009). Evidence for probiotic potential of a capsular-producing Streptococcus thermophilus CHCC 3534 strain. Polish journal of microbiology / Polskie Towarzystwo Mikrobiologów = The Polish Society of Microbiologists, 58(1), 49-55. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19469286
Kim, H. G., Kim, N.-R., Gim, M. G., Lee, J. M., Lee, S. Y., Ko, M. Y., Kim, J. Y., et al. (2008). Lipoteichoic acid isolated from Lactobacillus plantarum inhibits lipopolysaccharide-induced TNF-alpha production in THP-1 cells and endotoxin shock in mice. Journal of immunology (Baltimore, Md. : 1950), 180(4), 2553-61. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/18250466
Kim, H. G., Lee, S. Y., Kim, N. R., Lee, H. Y., Ko, M. Y., Jung, B. J., Kim, C. M., et al. (2011). Lactobacillus plantarum lipoteichoic acid down-regulated Shigella flexneri peptidoglycan-induced inflammation. Molecular immunology, 48(4), 382-91. doi:10.1016/j.molimm.2010.07.011
Kimmel, M., Keller, D, Farmer, S, & Warrino, D. E. (2010). A controlled clinical trial to evaluate the effect of GanedenBC(30) on immunological markers. Methods and findings in experimental and clinical pharmacology, 32(2), 129-32. doi:10.1358/mf.2010.32.2.1423881
Knap, I., Kehlet, A. B., Bennedsen, M., Mathis, G. F., Hofacre, C. L., Lumpkins, B. S., Jensen, M. M., et al. (2011). Bacillus subtilis (DSM17299) significantly reduces Salmonella in broilers. Poultry science, 90(8), 1690-4. doi:10.3382/ps.2010-01056
Kolida, S., Tuohy, K., & Gibson, G. R. (2007). Prebiotic effects of inulin and oligofructose. British Journal of Nutrition, 87(S2), S193. doi:10.1079/BJN/2002537
Kramar’, L. V. (2002). [The intestinal microenvironment in healthy persons under the technogenic action of an industrial city]. Vestnik Rossiĭskoĭ akademii meditsinskikh nauk / Rossiĭskaia akademiia meditsinskikh nauk, (8), 37-40. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12212377
Kumar, M., Kumar, A., Nagpal, R., Mohania, D., Behare, P., Verma, V., Kumar, P., et al. (2010). Cancer-preventing attributes of probiotics: an update. International journal of food sciences and nutrition, 61(5), 473-96. doi:10.3109/09637480903455971
Kvietys, P. R., Twohig, B., Danzell, J., & Specian, R. D. (1990). Ethanol-induced injury to the rat gastric mucosa. Role of neutrophils and xanthine oxidase-derived radicals. Gastroenterology, 98(4), 909-20. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2311875
Ledochowski, M., Widner, B., Bair, H., Probst, T., & Fuchs, D. (2000). Fructose- and sorbitol-reduced diet improves mood and gastrointestinal disturbances in fructose malabsorbers. Scandinavian journal of gastroenterology, 35(10), 1048-52. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11099057
Ledochowski, M., Widner, B., Murr, C., Sperner-Unterweger, B., & Fuchs, D. (2001). Fructose malabsorption is associated with decreased plasma tryptophan. Scandinavian journal of gastroenterology, 36(4), 367-71. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11336160
Leser, T. D., Knarreborg, A., & Worm, J. (2008). Germination and outgrowth of Bacillus subtilis and Bacillus licheniformis spores in the gastrointestinal tract of pigs. Journal of applied microbiology, 104(4), 1025-33. doi:10.1111/j.1365-2672.2007.03633.x
Leshchuk, S. I., Popkova, S. M., Budnikova, Z. I., & Ochirzhapova, D. T. (2011). [Enteric microbiocenosis in the population of an industrial city]. Gigiena i sanitariia, Mar(2), 31-5. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/21598641
Ling, W. H., Korpela, R., Mykkänen, H., Salminen, S., & Hänninen, O. (1994). Lactobacillus strain GG supplementation decreases colonic hydrolytic and reductive enzyme activities in healthy female adults. The Journal of nutrition, 124(1), 18-23. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8283290
Liong, M. T., & Shah, N. P. (2006). Effects of a Lactobacillus casei synbiotic on serum lipoprotein, intestinal microflora, and organic acids in rats. Journal of dairy science, 89(5), 1390-9. doi:10.3168/jds.S0022-0302(06)72207-X
Liu, C. F., Tung, Y. T., Wu, C. L., Lee, B.-H., Hsu, W.-H., & Pan, T. M. (2011). Antihypertensive effects of Lactobacillus-fermented milk orally administered to spontaneously hypertensive rats. Journal of agricultural and food chemistry, 59(9), 4537-43. doi:10.1021/jf104985v
Liu, J.-E., Zhang, Y., Zhang, J., Dong, P.-L., Chen, M., & Duan, Z.-P. (n.d.). Probiotic yogurt effects on intestinal flora of patients with chronic liver disease. Nursing research, 59(6), 426-32. doi:10.1097/NNR.0b013e3181fa4dc6
Liévin, V., Peiffer, I., Hudault, S., Rochat, F., Brassart, D., Neeser, J. R., & Servin, A. L. (2000). Bifidobacterium strains from resident infant human gastrointestinal microflora exert antimicrobial activity. Gut, 47(5), 646-52. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1728100&tool=pmcentrez&rendertype=abstract
Lomax, A. R., & Calder, P. C. (2009). Prebiotics, immune function, infection and inflammation: a review of the evidence. The British journal of nutrition, 101(5), 633-58. doi:10.1017/S0007114508055608
Mandel, D. R., Eichas, K., & Holmes, J. (2010). Bacillus coagulans: a viable adjunct therapy for relieving symptoms of rheumatoid arthritis according to a randomized, controlled trial. BMC complementary and alternative medicine, 10, 1. doi:10.1186/1472-6882-10-1
Maragkoudakis, P. A., Chingwaru, W., Gradisnik, L., Tsakalidou, E., & Cencic, A. (2010). Lactic acid bacteria efficiently protect human and animal intestinal epithelial and immune cells from enteric virus infection. International journal of food microbiology, 141 Suppl, S91-7. doi:10.1016/j.ijfoodmicro.2009.12.024
Mayer, E. a. (2011). Gut feelings: the emerging biology of gut-brain communication. Nature reviews. Neuroscience, 12(August). Nature Publishing Group. doi:10.1038/nrn3071
Mañé, J., Pedrosa, E., Lorén, V., Gassull, M. A., Espadaler, J., Cuñé, J., Audivert, S., et al. (n.d.). A mixture of Lactobacillus plantarum CECT 7315 and CECT 7316 enhances systemic immunity in elderly subjects. A dose-response, double-blind, placebo-controlled, randomized pilot trial. Nutrición hospitalaria : organo oficial de la Sociedad Española de Nutrición Parenteral y Enteral, 26(1), 228-35. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/21519752
Meng, Q. W., Yan, L., Ao, X., Zhou, T. X., Wang, J. P., Lee, J. H., & Kim, I. H. (2010). Influence of probiotics in different energy and nutrient density diets on growth performance, nutrient digestibility, meat quality, and blood characteristics in growing-finishing pigs. Journal of animal science, 88(10), 3320-6. doi:10.2527/jas.2009-2308
Mitchell, H. (2006). Digestive Health. In H. Mitchell (Ed.), Sweeteners and Sugar Alternatives in Food Technology (pp. 44-51). Ames, Iowa: Blackwell Publishing.
Mohan, J. C., Arora, R., & Khalilullah, M. (1990). Preliminary observations on effect of Lactobacillus sporogenes on serum lipid levels in hypercholesterolemic patients. The Indian journal of medical research, 92, 431-2. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2079358
Molina, V. C., Médici, M., Taranto, M. P., & Font de Valdez, G. (2009). Lactobacillus reuteri CRL 1098 prevents side effects produced by a nutritional vitamin B deficiency. Journal of applied microbiology, 106(2), 467-73. doi:10.1111/j.1365-2672.2008.04014.x
Moreau, M. C., & Gaboriau-Routhiau, V. (1996). The absence of gut flora, the doses of antigen ingested and aging affect the long-term peripheral tolerance induced by ovalbumin feeding in mice. Research in immunology, 147(1), 49-59. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8739328
Naidu, A. S., Xie, X., Leumer, D. A., Harrison, S., Burrill, M. J., & Fonda, E. A. (2002). Reduction of sulfide, ammonia compounds, and adhesion properties of Lactobacillus casei strain KE99 in vitro. Current microbiology, 44(3), 196-205. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11821928
Naruszewicz, Marek, Johansson, M.-L., Zapolska-Downar, D., & Bukowska, Hanna. (2002). Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. The American journal of clinical nutrition, 76(6), 1249-55. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12450890
Neufeld, K. M., Kang, N., Bienenstock, J, & Foster, J. A. (2011). Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society, 23(3), 255-64, e119. doi:10.1111/j.1365-2982.2010.01620.x
Neufeld, K.-A., & Foster, J. a. (2009). Effects of gut microbiota on the brain: implications for psychiatry. Journal of psychiatry & neuroscience : JPN, 34(3), 230-1. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2674977&tool=pmcentrez&rendertype=abstract
Niedzielin, K., Kordecki, H., & Birkenfeld, B. (2001). A controlled, double-blind, randomized study on the efficacy of Lactobacillus plantarum 299V in patients with irritable bowel syndrome. European journal of gastroenterology & hepatology, 13(10), 1143-7. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11711768
Nomoto, K. (2005). Prevention of infections by probiotics. Journal of bioscience and bioengineering, 100(6), 583-92. doi:10.1263/jbb.100.583
Ogita, T., Tanii, Y., Morita, H., Suzuki, T., & Tanabe, S. (2011). Suppression of Th17 response by Streptococcus thermophilus ST28 through induction of IFN-γ. International journal of molecular medicine, 28(5), 817-22. doi:10.3892/ijmm.2011.755
Ott, S. J., Musfeldt, M., Wenderoth, D. F., Hampe, J, Brant, O., Fölsch, U. R., Timmis, K. N., et al. (2004). Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut, 53(5), 685-93. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1774050&tool=pmcentrez&rendertype=abstract
Ouwehand, A. C., Tiihonen, K., Saarinen, M., Putaala, H., & Rautonen, N. (2009). Influence of a combination of Lactobacillus acidophilus NCFM and lactitol on healthy elderly: intestinal and immune parameters. The British journal of nutrition, 101(3), 367-75. doi:10.1017/S0007114508003097
O’Mahony, S. M., Marchesi, J. R., Scully, P., Codling, C., Ceolho, A.-M., Quigley, E. M. M., Cryan, J. F., et al. (2009). Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biological psychiatry, 65(3), 263-7. doi:10.1016/j.biopsych.2008.06.026
O’Malley, D., Dinan, T. G., & Cryan, J. F. (2011). Altered expression and secretion of colonic interleukin-6 in a stress-sensitive animal model of brain-gut axis dysfunction. Journal of neuroimmunology, 235(1-2), 48-55. doi:10.1016/j.jneuroim.2011.04.003
Pagnini, C., Corleto, V. D., Hoang, S. B., Saeed, R., Cominelli, F., & Delle Fave, G. (2008). Commensal bacteria and “oncologic surveillance”: suggestions from an experimental model. Journal of clinical gastroenterology, 42 Suppl 3, S193-6. doi:10.1097/MCG.0b013e31817f1284
Paik, I. H., Toh, K. Y., Lee, C., Kim, J. J., & Lee, S. J. (2000). Psychological stress may induce increased humoral and decreased cellular immunity. Behavioral medicine (Washington, D.C.), 26(3), 139-41. doi:10.1080/08964280009595761
Park, C. S., Ihm, S.-H., Yoo, K.-D., Kim, D.-B., Lee, J.-M., Kim, H.-Y., Chung, W.-S., et al. (2010). Relation between C-reactive protein, homocysteine levels, fibrinogen, and lipoprotein levels and leukocyte and platelet counts, and 10-year risk for cardiovascular disease among healthy adults in the USA. The American journal of cardiology, 105(9), 1284-8. doi:10.1016/j.amjcard.2009.12.045
Park, D.-Y., Ahn, Y.-T., Huh, C.-S., Jeon, S.-M., & Choi, M.-S. (2011). The inhibitory effect of Lactobacillus plantarum KY1032 cell extract on the adipogenesis of 3T3-L1 Cells. Journal of medicinal food, 14(6), 670-5. doi:10.1089/jmf.2010.1355
Petrof, E. O., Claud, E. C., Sun, J., Abramova, T., Guo, Y., Waypa, T. S., He, S.-M., et al. (2009). Bacteria-free solution derived from Lactobacillus plantarum inhibits multiple NF-kappaB pathways and inhibits proteasome function. Inflammatory bowel diseases, 15(10), 1537-47. doi:10.1002/ibd.20930
Picard, C., Fioramonti, J., Francois, a, Robinson, T., Neant, F., & Matuchansky, C. (2005). Review article: bifidobacteria as probiotic agents — physiological effects and clinical benefits. Alimentary pharmacology & therapeutics, 22(6), 495-512. doi:10.1111/j.1365-2036.2005.02615.x
Pirzer, U., Schönhaar, A., Fleischer, B., Hermann, E., & Meyer zum Büschenfelde, K. H. (1991). Reactivity of infiltrating T lymphocytes with microbial antigens in Crohn’s disease. Lancet, 338(8777), 1238-9. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1682646
Pompei, A., Cordisco, L., Amaretti, A., Zanoni, S., Matteuzzi, D., & Rossi, Maddalena. (2007). Folate production by bifidobacteria as a potential probiotic property. Applied and environmental microbiology, 73(1), 179-85. doi:10.1128/AEM.01763-06
Pope III, C. A. (2002). Lung Cancer, Cardiopulmonary Mortality, and Long-term Exposure to Fine Particulate Air Pollution. JAMA: The Journal of the American Medical Association, 287(9), 1132-1141. doi:10.1001/jama.287.9.1132
Reikvam, D. H., Erofeev, A., Sandvik, A., Grcic, V., Jahnsen, F. L., Gaustad, P., McCoy, K. D., et al. (2011). Depletion of murine intestinal microbiota: effects on gut mucosa and epithelial gene expression. PloS one, 6(3), e17996. doi:10.1371/journal.pone.0017996
Roberfroid, M., Gibson, Glenn R, Hoyles, Lesley, McCartney, Anne L, Rastall, R., Rowland, I., Wolvers, D., et al. (2010). Prebiotic effects: metabolic and health benefits. The British journal of nutrition, 104 Suppl (E-S1), S1-63. doi:10.1017/S0007114510003363
Rodríguez, C., Medici, M., Mozzi, F., & Font de Valdez, Graciela. (2010). Therapeutic effect of Streptococcus thermophilus CRL 1190-fermented milk on chronic gastritis. World journal of gastroenterology : WJG, 16(13), 1622-30. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2848370&tool=pmcentrez&rendertype=abstract
Sandek, A., Anker, S. D., & von Haehling, S. (2009). The gut and intestinal bacteria in chronic heart failure. Current drug metabolism, 10(1), 22-8. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19149510
Sandek, A., Rauchhaus, M., Anker, S. D., & von Haehling, S. (2008). The emerging role of the gut in chronic heart failure. Current opinion in clinical nutrition and metabolic care, 11(5), 632-9. doi:10.1097/MCO.0b013e32830a4c6e
Sanders, M. E., Morelli, L., & Tompkins, T. A. (2003). Sporeformers as Human Probiotics : Bacillus , and Brevibacillus. Comprehensive Reviews In Food Science And Food Safety, 2, 101-110.
Sapranauskas, R., Gasiunas, G., Fremaux, C., Barrangou, R., Horvath, P., & Siksnys, V. (2011). The Streptococcus thermophilus CRISPR/Cas system provides immunity in Escherichia coli. Nucleic acids research. doi:10.1093/nar/gkr606
Satish Kumar, R., Kanmani, P., Yuvaraj, N., Paari, K. A., Pattukumar, V., & Arul, V. (2011). Lactobacillus plantarum AS1 binds to cultured human intestinal cell line HT-29 and inhibits cell attachment by enterovirulent bacterium Vibrio parahaemolyticus. Letters in applied microbiology, 53(4), 481-7. doi:10.1111/j.1472-765X.2011.03136.x
Savage, D. C. (1977). Microbial ecology of the gastrointestinal tract. Annual review of microbiology, 31, 107-33. doi:10.1146/annurev.mi.31.100177.000543
Savilov, E. D., & Shcherbakova, E. B. (2003). [Acute intestinal infections in children in areas with industrial environmental air pollution]. Gigiena i sanitariia, Aug(4), 6-8. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12934271
Scarpellini, E., Campanale, M., Leone, D., Purchiaroni, F., Vitale, G., Lauritano, E. C., & Gasbarrini, A. (2010). Gut microbiota and obesity. Internal and emergency medicine, 5 Suppl 1, S53-6. doi:10.1007/s11739-010-0450-1
Selvam, R., Maheswari, P., Kavitha, P., Ravichandran, M., Sas, B., & Ramchand, C. N. (2009). Effect of Bacillus subtilis PB6, a natural probiotic on colon mucosal inflammation and plasma cytokines levels in inflammatory bowel disease. Indian journal of biochemistry & biophysics, 46(1), 79-85. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19374258
Shimosato, T., Tohno, M., Sato, T., Nishimura, J., Kawai, Y., Saito, T., & Kitazawa, H. (2009). Identification of a potent immunostimulatory oligodeoxynucleotide from Streptococcus thermophilus lacZ. Animal science journal = Nihon chikusan Gakkaihō, 80(5), 597-604. doi:10.1111/j.1740-0929.2009.00680.x
Simon, G. L., & Gorbach, S. L. (1984). Intestinal flora in health and disease. Gastroenterology, 86(1), 174-93. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6357937
Singh, J., Rivenson, A., Tomita, M., Shimamura, S., Ishibashi, N., & Reddy, B. S. (1997). Bifidobacterium longum, a lactic acid-producing intestinal bacterium inhibits colon cancer and modulates the intermediate biomarkers of colon carcinogenesis. Carcinogenesis, 18(4), 833-41. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9111222
So, J.-S., Song, M.-K., Kwon, H.-K., Lee, C.-G., Chae, C.-S., Sahoo, A., Jash, A., et al. (2011). Lactobacillus casei enhances type II collagen/glucosamine-mediated suppression of inflammatory responses in experimental osteoarthritis. Life sciences, 88(7-8), 358-66. doi:10.1016/j.lfs.2010.12.013
Stephani, J., Radulovic, K., & Niess, J. H. (2011). Gut microbiota, probiotics and inflammatory bowel disease. Archivum immunologiae et therapiae experimentalis, 59(3), 161-77. doi:10.1007/s00005-011-0122-5
Subramanya, S. B., Subramanian, V. S., & Said, H. M. (2010). Chronic alcohol consumption and intestinal thiamin absorption: effects on physiological and molecular parameters of the uptake process. American journal of physiology. Gastrointestinal and liver physiology, 299(1), G23-31. doi:10.1152/ajpgi.00132.2010
Sudo, N., Chida, Y., Aiba, Y., Sonoda, J., Oyama, N., Yu, X.-N., Kubo, C., et al. (2004). Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. The Journal of physiology, 558(Pt 1), 263-75. doi:10.1113/jphysiol.2004.063388
Swidsinski, A., Ladhoff, A., Pernthaler, A., Swidsinski, S., Loening-Baucke, V., Ortner, M., Weber, J., et al. (2002). Mucosal flora in inflammatory bowel disease. Gastroenterology, 122(1), 44-54. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11781279
Taguchi, H., Takahashi, M., Yamaguchi, H., Osaki, T., Komatsu, A., Fujioka, Y., & Kamiya, S. (2002). Experimental infection of germ-free mice with hyper-toxigenic enterohaemorrhagic Escherichia coli O157:H7, strain 6. Journal of medical microbiology, 51(4), 336-43. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11926740
Takemura, N., Okubo, T., & Sonoyama, K. (2010a). Lactobacillus plantarum strain No. 14 reduces adipocyte size in mice fed high-fat diet. Experimental biology and medicine (Maywood, N.J.), 235(7), 849-56. doi:10.1258/ebm.2010.009377
Takemura, N., Okubo, T., & Sonoyama, K. (2010b). Lactobacillus plantarum strain No. 14 reduces adipocyte size in mice fed high-fat diet. Experimental biology and medicine (Maywood, N.J.), 235(7), 849-56. doi:10.1258/ebm.2010.009377
Tanaka, K., & Ishikawa, H. (2004). Role of intestinal bacterial flora in oral tolerance induction. Histology and histopathology, 19(3), 907-14. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15168353
Tlaskalová-Hogenová, H., Stepánková, R., Hudcovic, T., Tucková, L., Cukrowska, B., Lodinová-Zádníková, R., Kozáková, H., et al. (2004). Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunology letters, 93(2-3), 97-108. doi:10.1016/j.imlet.2004.02.005
Tuohy, K. M., Pinart-Gilberga, M., Jones, M., Hoyles, L, McCartney, A L, & Gibson, G R. (2007). Survivability of a probiotic Lactobacillus casei in the gastrointestinal tract of healthy human volunteers and its impact on the faecal microflora. Journal of applied microbiology, 102(4), 1026-32. doi:10.1111/j.1365-2672.2006.03154.x
Usman, & Hosono, A. (2000). Effect of administration of Lactobacillus gasseri on serum lipids and fecal steroids in hypercholesterolemic rats. Journal of dairy science, 83(8), 1705-11. doi:10.3168/jds.S0022-0302(00)75039-9
Vicario, M., Alonso, C., Guilarte, M., Serra, J., Martínez, C., González-Castro, A. M., Lobo, B., et al. (2011). Chronic psychosocial stress induces reversible mitochondrial damage and corticotropin-releasing factor receptor type-1 upregulation in the rat intestine and IBS-like gut dysfunction. Psychoneuroendocrinology, Epub ahead. doi:10.1016/j.psyneuen.2011.05.005
de Vrese, M., Winkler, P., Rautenberg, P., Harder, T., Noah, C., Laue, C., Ott, S., et al. (2005). Effect of Lactobacillus gasseri PA 16/8, Bifidobacterium longum SP 07/3, B. bifidum MF 20/5 on common cold episodes: a double blind, randomized, controlled trial. Clinical nutrition (Edinburgh, Scotland), 24(4), 481-91. doi:10.1016/j.clnu.2005.02.006
Wang, H. J., Zakhari, S., & Jung, M. K. (2010). Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development. World journal of gastroenterology : WJG, 16(11), 1304-13. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2842521&tool=pmcentrez&rendertype=abstract
Wang, X., Wang, B.-R., Zhang, X.-J., Xu, Z., Ding, Y.-Q., & Ju, G. (2002). Evidences for vagus nerve in maintenance of immune balance and transmission of immune information from gut to brain in STM-infected rats. World journal of gastroenterology : WJG, 8(3), 540-5. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12046088
Williams, P. (2007). Bacillus subtilis: a shocking message from a probiotic. Cell host & microbe, 1(4), 248-9. doi:10.1016/j.chom.2007.05.010
Willing, B. P., Russell, S. L., & Finlay, B. B. (2011). Shifting the balance: antibiotic effects on host-microbiota mutualism. Nature reviews. Microbiology, 9(4), 233-43. doi:10.1038/nrmicro2536
Wong, J. M. W., de Souza, R., Kendall, C. W. C., Emam, A., & Jenkins, D. J. A. (2006). Colonic health: fermentation and short chain fatty acids. Journal of clinical gastroenterology, 40(3), 235-43. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16633129
Yasui, H., Kiyoshima, J., & Ushijima, H. (1995). Passive protection against rotavirus-induced diarrhea of mouse pups born to and nursed by dams fed Bifidobacterium breve YIT4064. The Journal of infectious diseases, 172(2), 403-9. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7622883
Younes, H., Coudray, C., Bellanger, J., Demigné, C., Rayssiguier, Y., & Rémésy, C. (2001). Effects of two fermentable carbohydrates (inulin and resistant starch) and their combination on calcium and magnesium balance in rats. The British journal of nutrition, 86(4), 479-85. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11591235
Yuki, N., Watanabe, K., Mike, A., Tagami, Y., Tanaka, R., Ohwaki, M., & Morotomi, M. (1999). Survival of a probiotic, Lactobacillus casei strain Shirota, in the gastrointestinal tract: selective isolation from faeces and identification using monoclonal antibodies. International journal of food microbiology, 48(1), 51-7. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10375134
Zareie, M., Johnson-Henry, K., Jury, J., Yang, P.-C., Ngan, B.-Y., McKay, D. M., Soderholm, J. D., et al. (2006). Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut, 55(11), 1553-60. doi:10.1136/gut.2005.080739
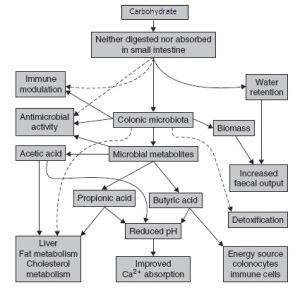
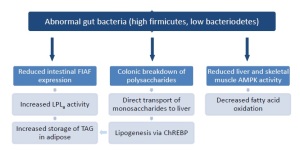
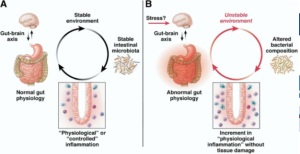
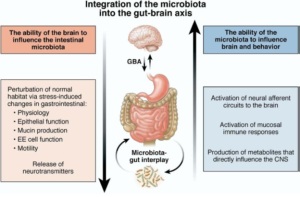
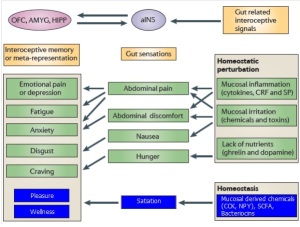
I blog quite often and I genuinely thank you for your information. Your
article has really peaked my interest. I will take a
note of your site and keep checking for new information about once per
week. I opted in for your Feed as well.